Manufacturer and partners to produce 1m COVID swabs a day – Med-Tech Innovation | Latest news for the medical device industry
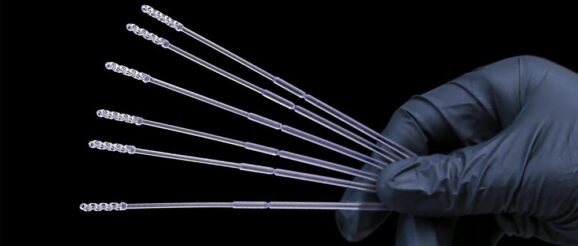
EnvisionTEC, a manufacturer of desktop and full-production 3D printers and materials, has completed a clinical trial to use the EnvisionTEC 3D printed Nasopharyngeal (NP) Swabs for COVID-19 testing.
EnvisionTEC, as well as a growing number of its Envision One cDLM customers who have also registered with the FDA to take part in this endeavour.
Many of the owners of these units are accustomed to producing medical-grade products, making them the ideal production partners for this enterprise. The Envision One is capable of producing up to 2400 swabs in 24 hours. This leads to a production capacity of EnvisionTEC and its Envision One user network of up to a million swabs per day.
The company worked with Beth Israel Deaconess Medical Center (BIDMC) to develop a swab design and material to be printed on its Envision One cDLM 3D printer.
There are over a thousand units of the Envision One currently in use among dental labs, orthodontic practices, universities and medical device manufacturers.
EnvisionTEC engineers have designed a collection tip for a flexible nasal swab that has completed testing in an IRB-approved clinical trial for use in this unprecedented time.
Mechanical and chemical testing of both the design and the material was done to ensure that the swabs pick up viral RNA particles and do not interfere with PCA/reagents, that they are chemically safe, that they would bend 180 degrees without breaking, and that the design would be able to safely collect enough virus particles from the nasal passage to effectively test. The EnvisionTEC NP swab continued to perform in the same way mechanically after being sterilised by steam at 270°F at 27 Pa in an autoclave.
Dr. Ramy Arnaout, associate director of the clinical microbiology laboratories at Beth Israel Deaconess Medical Centre, said: “Analytical results were positive, with a high level of concordance with the reference swab and with subjective results showing the swab performed neutrally or better than other test swabs.”
