Tracking Healthcare Innovation Globally: Fighting The Pandemic
Vasudev Bailey, PhD, Zoe Guttendorf, ARTIS VENTURES
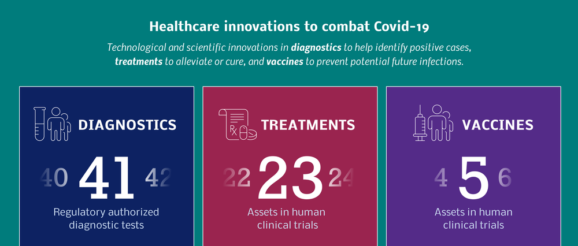
Scientists, clinicians, and researchers across the globe are working tirelessly to fight the pandemic, focusing efforts on three main areas of development:
Diagnostics, wherein we can identify both positive and negative COVID-19 patients
Treatments for those infected, which will help reduce symptoms, prevent complications, and potentially even cure COVID-19
Vaccines, which when administered will help in fighting off the viral infection.
It’s very encouraging to see efforts emerging from every pocket of the world as we all strive to fight this pandemic on a global scale, and all three categories already have significant work both in development, and in the preclinical pipeline.
We have compiled an innovation tracker at www.av.co/covid to keep tabs on the progress. We will keep this updated regularly.
The diagnostics innovation and approval is fortunately a long list, which makes the market quite fragmented. We chatted with a few provider leaders, looking to ascertain if there was a leading market choice, with the resulting takeaway being that they were less concerned about timeliness of test results, and more concerned (rightfully so, perhaps) about sensitivity of these tests. This means they want to be sure there are limited “false negatives” (bottom left quadrant) so that people who actually DO have COVID-19 think they don’t, and then continue walking around, not practicing the appropriate social distancing and quarantine directives, and continue the spread of the virus to others.
In the world of diagnostics, while there are several products in development, our focus was to rely on data from regulatory bodies to help in ensuring that the sensitivity of the tests aren’t compromised. Our data was determined by:
FDA Approved diagnostics have undergone regulatory protocol to determine that the device is safe and effective. This process can take months to years, depending on the complexity of the test. No COVID-19 diagnostic test has been FDA-approved to date.
FDA Emergency Use Authorization (EUA) can be granted to unapproved devices in the case of emergencies to diagnose, treat, or prevent serious conditions when public health threats arise. As of 3/30/20, 20 diagnostics were granted EUA to detect COVID-19.
CE Mark denotes that products comply with relevant health, safety, and environmental standards to be sold in the European market.
Laboratory Developed Tests (LDT) are manufactured and used within a single lab. LDTs are used similarly to FDA approved tests, but labs may choose to use their own test even when another option is available on the market. These tests don’t always need to undergo the same FDA requirements/protocol, as they are often relatively simple lab tests.
WHO Emergency Use Listing (EUL) procedure is used during public health emergencies to get unapproved diagnostics to impact patients faster. EUL is assessed based on a minimum set of available quality, safety, and performance data.
While we don’t have a good sense of the real world sensitivity and specificity of these diagnostics tests, what is clear is that there is no gold standard test, and many of these tests listed below are used across the world depending on what reagents are available and what machines (if PCR based) they have available.
For a full list of diagnostics, click here: www.av.co/covid-diagnostics
The likelihood is that of all the innovations to fight this pandemic, the biggest impact that can be made will be from a pharma/biotech company that has an agent that is able to prevent viral growth, reduce hospitalization, reduce complications from having been infected, or will cure the person afflicted with COVID-19. As the world drug developers put their heads down and work on this solution, a creative path here has been the use of repurposed drugs that may prove to be efficacious in treating COVID-19.
For a full list of drugs and therapies that are in human trials, click here: https://www.av.co/covid-treatments
It is highly unlikely that a commercially viable vaccine will be ready over the next six months, or even in the next year. While Moderna seems to lead the pack in the US and a modified BCG vaccine is being developed in Europe and Australia, all claim that they may have a vaccine this summer. The challenge though isn’t as quite easy as they describe and even iIf successful, may only be available to at-risk health workers first. The development of a quick vaccine will require rapidly overcoming a variety of solutions from understanding immunological response and identifying antigens that generate protective response (easier challenges with technology, data and AI) to solving pathogen variability, variable genetic response, functional response, short effector memory or environmental factors and response (harder challenges due to lack of data and tools to be predictive and the need for real world evidence).
For a full list of vaccines that are in human testing, click here: www.av.co/covid-vaccines
LET’S KEEP THIS GOING!
We have worked to compile the available diagnostics as well the vaccines and therapies/drugs that are currently in patient trials. We’re also keeping an eye on early clinical projects. We’ll be updating www.av.co/covid regularly. Please message us if you are aware of any other projects/studies not included here. We are optimistic that the healthcare community at large will come together to find an end to COVID-19.
Resource list available upon request.
