Surface innovation in spinal technology
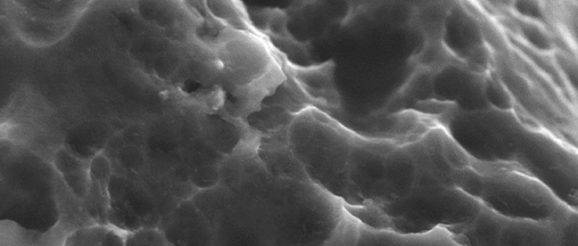
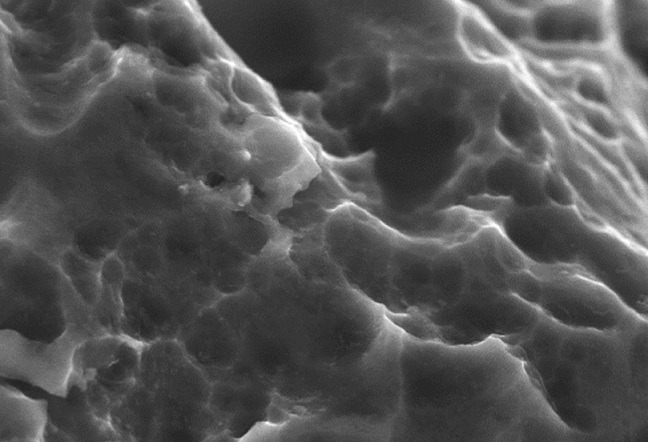
Surface technology for interbody fusion implants is an area of massive growth in the spinal market, with a large number of device companies and academic institutions hunting for the best ways to optimise bone/device integration. Some have focused on micro-, macro- and nanoscale textures, some on additive titanium sprays, and some on 3D-printed porous metal surfaces. Spinal News International speaks to physicians Richard Guyer (Plano, USA) and Paul Slosar (San Franscisco, USA), to scientist Barbara Boyan (Richmond, USA), and to engineer Sam Fang (Dallas, USA), about the latest developments in the field.
How has interbody fusion technology developed over the past couple of decades?
Guyer: In the past, we would use allograft or autologous bone graft during interbody fusions or fusion procedures in general. It takes a long time, however, for bone cells to actually resorb some of these substances and begin forming new bone.
Slosar: We used to be taught that the interbody fusion device was simply a spacer—there to deliver biologic material. The idea of early healing was somewhat a second thought as it pertains to the device. Much more, people were focused on what you were putting in the device to make the bone heal.
Guyer: We progressed to using titanium because it is a substrate which cells will grow on to. But, the problem with pure titanium implants is that they cause a lot of interference when you take X-rays—there are a lot of artefacts, and you really cannot see well because you have this lump of metal, making it hard to assess fusion.
Slosar: Early titanium devices, from around 20 years ago, were very smooth titanium—there were no specific surface characteristics to them; they basically looked like shotgun shells.
Guyer: When I think back to original metal implants, they were very, very dense. The newer ones are less dense, with less metal particles in them, so they may be okay. In the past, the devices were very stiff so, if one put them in between the vertebrae, they could cause subsidence.
Boyan: Subsidence was a problem caused by the design of certain implants, rather than the use of titanium itself. This occurred in some cases because of both thin designs and surgical approach. Today, implants are still “stiff”, but designs have improved to reduce the possibility of subsidence.
Guyer: Somehow, someone came up with the idea for polyether ether ketone (PEEK), which is a radiolucent plastic-like material. PEEK can X-ray very well, and it has a similar structure to bone so it is less likely to subside. The problem with PEEK, however, is that it is hydrophobic; bone cells will not grow to it. In the biomechanical research I have been involved with, we found that bone did not grow to PEEK.1
Boyan: Bone is a tissue that is constantly being remodelled. Certain bone cells are constantly resorbing tissue (osteoclasts), while others are constantly making new bone (osteoblasts). This is what keeps it healthy and resilient. Osteoblasts come from less-differentiated osteoprogenitor cells. When those cells see PEEK, they do not choose to become bone cells; instead they choose to become more like fibroblasts. They form fibrous layers of tissue right next to the PEEK. It is biocompatible, but also a material that causes cells to become more fibrous-like and less bone-cell like. Tantalum is has a similar effect. When cells are on a tantalum surface they attach and they multiply, but again they are not going to differentiate as well into an osteoblast. In contrast, when osteoprogenitor cells are on titanium implant surfaces, they differentiate into osteoblasts.
Guyer: The next progression in implant design was to put titanium onto PEEK implants. In the research I was involved in, we found that bone cells are attracted to these surfaces, and that is where the osseointegration comes from.1 If you can use an implant that the bone will grow to, then you increase the chance of fusion. Typically, fusion rates are relatively high, but increasing any surface area between the implant and the bone will bring another source of cell growth and so, stability.
Fang: I think that interbody implant design has really changed quite a bit over the past three to four years. Just a few years ago, we only really had PEEK. Now, we have a bunch of 3D-printed metal devices—it is really improving.
Can adding titanium to PEEK solve the problems associated with PEEK-only and early titanium implants?
Boyan: If you were to coat a PEEK surface with titanium you might temporarily get the advantages of titanium. But, titanium does not stick very well to PEEK. When the surgeon goes to put it in the body under mechanical force some of that surface will come off. It just does not adhere as well to PEEK as everybody would love it to.
Guyer: There are different ways of making titanium coatings—either spraying it on in an additive process or using a subtractive process in which the titanium may be acid-etched. There are spray coatings which have been associated with delamination of the titanium.2 This is not good for the fusion, and it is not good for the patient either.
Slosar: Delamination is a clinical concern for me, particularly with regards to late-stage problems. There was not always a great awareness of what was going to happen with, for example, total hip replacements when doctors began using cement or coatings on the surfaces of the implants. They got early fixation and good clinical results to begin with, but they did not anticipate later-stage problems with osteolysis.
In the long run, it appears that titanium wear debris may lead to clinical problems that are not yet well-described. I fear that there will be issues of chronic inflammation similar to those we have seen with osteolysis around early-stage total joint implants.
Guyer: No-one has ever observed titanium toxicity, however, we do know that large quantities of titanium can be toxic to the body. Titanium is generally very well-tolerated by the body in small amounts, but in a world where we are exposed to so many foreign elements—toxins in the environment and chemicals in the blood—we would like to avoid exposing the patient to it. As well as this, the simple principle remains that if you have a coating on an implant, you would like it to stay there; not to move or float around in the body by any means.
If implants are spray-coated with titanium, then I would like to know if the manufacturers have performed research into the incidence of delamination. If the coatings are deeply porous like the ones like we were studying in our experiment, then I would also feel much more secure that they will not delaminate.
In your opinion, what is the next big development for surface technology?
Guyer: Implants with porosity—in particular titanium—that can offer a bioactive surface. Instead of the surface being inactive, it can now actually interact with the bone, acting as another site for stability. As well as promoting fusion, implants made of porous titanium retain the advantages of a strong implant, while reducing the likelihood of collapsing into the bone by having a smaller volume of metal. This regular and repeatable nanosurface that can create a programmable biologic response, combined with a deeply porous surface that our team is researching, would seem to be an ideal solution.
In our research, bone grew to the deeply porous titanium we evaluated.1 Bone can grow into all of these little caverns and inter-digitating spaces like a locking interface, which results in better integration than from just the bone graft.
Slosar: I think that nanoscale technologies in medicine are going to be incredibly important, both for the delivery of medications to remote parts of the body, as well as creating technologically “smart” surfaces for orthopaedic implants. What is very clear from our literature is that if you want the most reliable, rapid, osseous integration—which in my opinion leads to a very rapid clinical result—a sophisticated sub-micron or nanoscale titanium surface is necessary to get a rapid integration between the host bone and the cage. The actual engagement between the sophisticated surface of the implant and the host bone being the key to the fusion; to stimulate rapid bone integration and set the stage for rapid clinical improvement.
Guyer: I am very much in favour of porous titanium coatings on PEEK implants. A variety of implants have screws in addition to titanium coatings, and we can use these knowing that we have a method of fixation bone to the implant, besides the screws. It is important to know how they are made, however.
We have gone from inner implants to bioactive implants that will actually interact with the bone, allowing bone to grow on them or even into them. This extra source of fixation will lessen the overall chance of non-union or failure of the fusion itself to heal.
Fang: I think the market is heading in the direction of changing traditional non-bioactive PEEK into a bioactive material. With PTC (PEEK titanium composite, Orthofix), we are trying to conserve the best properties of the PEEK—low stiffness and radiolucency. As an engineer, I believe this represents the future.
Orthofix’s PEEK Titanium Composite
Sam Fang—one of the engineers behind Orthofix’s PEEK Titanium Composite (PTC) technology—speaks to Spinal News International about the process of developing the technology, and its potential benefits.
What were your primary concerns when developing PTC?
Fang: Our major concern starting out was trying to combine porous titanium with PEEK. Manufacturing a device with these two materials is not easy—if the titanium is porous, when we try to injection-mould the PEEK in a molten state, it will penetrate the pores of the titanium, which renders it useless for bone growth.
We ended up with a design which included an intermediate layer of solid titanium, separating the PEEK and the porous layer. If you look at our titanium endplates, you can see that we actually have three layers—the top layer of porous titanium, the intermediate level, and then a bottom layer comprised of dovetails which allow for very strong mechanical bonding with the moulded PEEK.
How are the implants manufactured?
Fang: We use the latest 3D printing to create the very complex shape of our titanium endplates. Then, we cut the shape and use injection moulding to combine the titanium with PEEK. This is our basic process.
Of course, there are many nuances to this process—for example, we need to engineer the shape of the endplates precisely to make sure that they do not let the PEEK flow from the sides, and we need to make sure that the titanium does not oxidise after the cutting. We also use an acid etching process to create nano-scale surface features on the porous titanium endplates. Our latest study shows that these features are on the scale of 10 to 40 nanometers.
How exactly does this surface interact with bone?
Fang: The top layer of the titanium, which is in contact with the bone, is 50% porous. We know that porous titanium is good for bone ingrowth—the tricky part is making sure that we are optimising the size of the pore, and the porosity. We did some research, and found out that pore size at the 400 micron range and a porosity of 50% is optimal, and so that is where we set our parameters.
Most surface coating is too thin to offer a good pore depth. Most coatings probably have about 100 microns of depth, which means that bone can only really attach by ongrowth; it cannot really grow into the surface In our design, the pores have a depth of around 900 microns, which is roughly nine times that of most surface coatings. That is why we say our technology not only promotes ongrowth—we are also talking about ingrowth.
Is there any risk of delamination with this technology?
Fang: We have never seen delamination in our testing. The reason is quite simple—our technology is not really a surface coating. The dovetail mechanism provides super-strong mechanical locking between the titanium and the PEEK.
In our testing the first thing to fail is always the layer of pure PEEK—never the connecting layer. In other words, interconnection between the titanium and PEEK layer is actually stronger than the PEEK itself. I do not think that delamination is an issue for this type of design at all.
Are there any drawbacks at all to this technology?
Fang: The technology is not the best for very small implants. Some implants are only 4mm tall. Our titanium layers need to be thick to get as much bone ingrowth as possible, leaving a device of this size with only 1mm of space for the PEEK section, which is probably too thin.
Titan Spine’s Surface Technology and NanoLock
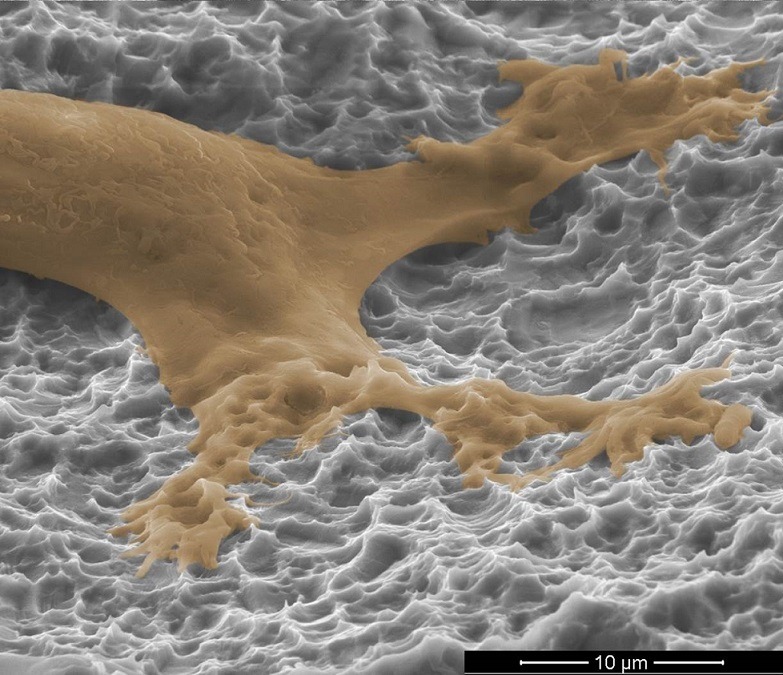
Paul Slosar, a physician who has been using Titan Spine’s titanium devices with macro-, micro- and cellular-level surface technology since 2008, and Barbara Boyan, a scientist involved in the design of the company’s next-generation Nanolock technology, discuss Titan Spine’s products and their place in the interbody fusion market.
Have you noticed better fusion rates since using these devices?
Slosar: Absolutely—there is no doubt in my mind. In general, I have seen patients starting to resume activities two to three months earlier than they used to with either allograft bone or PEEK. Patients feel more secure mechanically and clinically.
Have you experienced any drawbacks to using these devices?
Slosar: To answer your question on a clinical basis, I have not. They have allowed me to dramatically reduce my utilisation of high potency and expensive biologics, because the implant is actually participating in the fusion.
I also published a paper in 2015 looking at the issue of radiolucency.3 We reviewed 56 fusion segments and sent those cases out to two different radiologists. We had an 82% interobserver agreement across the board, and even higher percentages of agreement across certain select categories. The idea that you cannot see around these implants on an X-ray is not true. The Titan Spine cages, if anything, are even somewhat more generous, with large side-windows.
In my opinion, radiolucency is a little bit of a made-up issue. In clinical experience, whether you have a titanium spacer, a PEEK spacer or a cadaver bone, if you see any unusual signs on a patient’s X-ray that make you suspicious, you always get a computed tomography (CT) scan. If you are trying to determine if somebody has a successful fusion or a nonunion, the CT scan is the gold standard.
Doctors have become more comfortable not seeing their implant on imaging, like with PEEK, where you cannot see the implant at all. Sometimes doctors may even hide behind less-than-optimal carpentry—it looks okay because you cannot see it. When I have trained doctors on titanium-only implants, it has forced them to become better carpenters, because they will leave a visible implant.
How does the new Nanolock surface work?
Boyan: During normal bone remodelling, osteoclasts leave a little space behind that has a particular shape to it, which is attractive to osteoprogenitor cells. Our Nanolock surface mimics that shape to provide the best physical environment for these cells to differentiate into osteoblasts and form bone.
In general, all titanium surfaces are attractive to osteoblasts, but not all devices provide this shape-property on the surface that the osteoblasts like. The Nanolock surface, which is made by a proprietary subtractive method, is just more attractive.
In this particular case, the design of this surface really came from us in the science world. We were looking at titanium and how to make it better; all of it done in a university setting, without any conflict of interest. We went about the development in a very organised way—we started with 26 different versions of the surface to see which one was best. This surface was the winner.
Do you think that the future for spinal fusion devices lies in titanium?
Boyan: Titanium, in my mind, is really advantageous in bone, and titanium with an osteogenic surface is the way to go. Sometimes the manufacturing for other materials is less expensive, for example, but I think that titanium is really superior.
Slosar: Titan Spine implants have an absolutely unique surface characteristic. It is not just simple titanium—it is the sophisticated surface that has made the science. There is a significant difference in terms of the cellular response to these highly-specific nanoscale implants versus just a spray of titanium on a piece of plastic.
Smooth titanium is better than PEEK, sub-micron titanium is better than smooth, and nanoscale is better than micron alone, in terms of driving a cellular response towards bone formation. Nanotechnology, I think, is the future of orthopaedic surface technology.
References
- Guyer RD, Abitbol JJ, Ohnmeiss DD and Yao C, Spine (Phila Pa 1976) 2016. [Epub ahead of print] doi: 10.1097/BRS.0000000000001672
- Kienle A, Graf N and Wilke HJ, Spine J 2016; 16(2): 235-42
- Slosar PJ, et al, Am J Orthop (Belle Mead NJ) 2015; 44(2): 86-9
The post Surface innovation in spinal technology appeared first on Spinal News International.
