Injectable bioelectrodes with tunable lifetimes for neuromuscular diseases and neurological disorders – Innovation Toronto
The new graphene-based conductive hydrogel electrodes offer convenience of use, controllable degradation, and excellent signal transmission
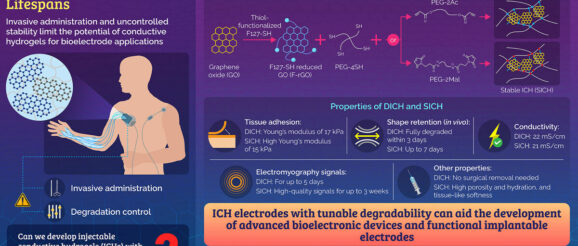
Implantable bioelectrodes are electronic devices that can monitor or stimulate biological activity by transmitting signals to and from living biological systems. Such devices can be fabricated using various materials and techniques. But, because of their intimate contact and interactions with living tissues, selection of the right material for performance and biocompatibility is crucial. In recent times, conductible hydrogels have attracted great attention as bioelectrode materials owing to their flexibility, compatibility, and excellent interaction ability. However, the absence of injectability and degradability in conventional conductive hydrogels limits their convenience of use and performance in biological systems.
Against this backdrop, researchers from Korea have now developed graphene-based conductive hydrogels possessing injectability and tunable degradability, furthering the design and development of advanced bioelectrodes. The study was led by Professor Jae Young Lee from Gwangju Institute of Science and Technology (GIST) and was published in the Small journal on 24 February 2023.
Explaining the rationale for their study, Prof. Lee says, “Traditional implantable electrodes frequently cause several problems, such as large incision for implantation and uncontrolled stability in the body. In contrast, conductive hydrogel materials allow minimally invasive delivery and control over the bioelectrode’s functional in vivo lifespan and are thus highly desired.”
To synthesize the injectable conductive hydrogels (ICHs), the researchers used thiol-functionalized reduced graphene oxide (F-rGO) as the conductive component due to its large surface area and excellent electrical and mechanical properties. They selected dimaleimide (PEG-2Mal)- and diacrylate (PEG-2Ac)-functionalized polyethylene glycol as prepolymers to facilitate the development of ICHs that are stable and hydrolysable, respectively. These prepolymers were then subjected to thiol-ene reactions with poly (ethylene glycol)-tetrathiol (PEG-4SH) and F-rGO.
ICHs made with PEG-2Ac were degradable (DICH), while those with PEG-2Mal were stable (SICH). The researchers found that the novel ICHs outperformed various existing ones by binding extremely well to tissues and recording the highest signals. Under in vitro conditions (outside a living organism), SICH did not degrade for a month, while DICH showed gradual degradation from the third day onwards.
When implanted onto mouse skin, DICH disappeared after three days of administration, whereas SICH retained its shape for up to 7 days. In addition to controlled degradability, both ICHs were skin-compatible.
Further, the team evaluated the ability of the ICHs to record in vivo electromyography signals in rat muscle and skin. Both SICH and DICH recorded high-quality signals and surpassed the performance of traditional metal electrodes. The SICH recordings could be monitored up to three weeks, whereas DICH signals were completely lost after five days. These findings suggest the applicability of SICH electrodes for long-term signal monitoring and that of DICH for temporary use requiring no surgical removal.
Summarising these results, Prof. Lee says, “The novel graphene-based ICH electrodes developed by us incorporate features like high signal sensitivity, simplicity of use, minimal invasiveness, and tunable degradability. Altogether, these properties can assist in the development of advanced bioelectronics and functional implantable bioelectrodes for a variety of medical conditions, such as neuromuscular diseases and neurological disorders.”
We certainly hope this development ushers in a new era of therapeutic and diagnostic advancement soon!
