Could solid-state refrigerants become a viable replacement for current air conditioning and other cooling technologies? – Innovation Toronto
Summer is in full swing in the U.S., and people are turning up their air conditioners to beat the heat. But the hydrofluorocarbon refrigerants in these and other cooling devices are potent greenhouse gases and major drivers of climate change. Today, scientists report a prototype device that could someday replace existing “A/Cs.” It’s much more environmentally friendly and uses solid refrigerants to efficiently cool a space.
The researchers will present their results today at the fall meeting of the American Chemical Society (ACS). ACS Fall 2022 is a hybrid meeting being held virtually and in-person Aug. 21–25, with on-demand access available Aug. 26–Sept. 9. The meeting features nearly 11,000 presentations on a wide range of science topics.
“Just installing an air conditioner or throwing one away is a huge driver of global warming,” says Adam Slavney, Ph.D., who is presenting this work at the meeting. The refrigerants used in these systems are thousands of times more potent than carbon dioxide and can accidentally leak out of systems when they are being handled or disposed of.
Traditional cooling systems, such as air conditioners, work by causing a refrigerant to cycle between being a gas or a liquid. When the liquid becomes a gas, it expands and absorbs heat, cooling a room or the interior of a refrigerator. A compressor that works at about 70–150 pounds per square inch (psi) turns the gas back into a liquid, releasing heat. In the case of air conditioners, this heat is directed outside the home. Though this cycle is efficient, concerns about climate change and stricter regulations on hydrofluorocarbon refrigerants are spurring the search for more environmentally responsible ones.
Solid refrigerants could be an ideal solution. Unlike gases, solids won’t leak into the environment from A/C units. One class of solid refrigerants, called barocaloric materials, work similarly to traditional gas-liquid cooling systems. They use pressure changes to go through heat cycles, but in this case, the pressure drives a solid-to-solid phase change. That means the material remains a solid, but the internal molecular structure changes. The key structural aspect of these barocaloric solid materials is that they contain long, flexible molecular chains that are typically floppy and disordered. But under pressure, the chains become more ordered and rigid — a change that releases heat. The process of going from an ordered to a relaxed structure is like melting wax, but without it becoming a liquid, says Jarad Mason, Ph.D., the project’s principal investigator, who is at Harvard University. When the pressure is released, the material reabsorbs heat, completing the cycle.
A disadvantage of barocaloric systems, however, is that most of these materials require massive pressures to drive heat cycles. To produce these pressures, the systems need expensive, specialized equipment that’s not practical for real-world cooling applications. Mason and his team recently reported barocaloric materials that can act as refrigerants at much lower pressures. They’ve now shown that the refrigerants, which are called metal-halide perovskites, can work in a cooling system they’ve built from scratch. “The materials we reported are able to cycle at about 3,000 psi, which are pressures that a typical hydraulics system can work at,” says Slavney.
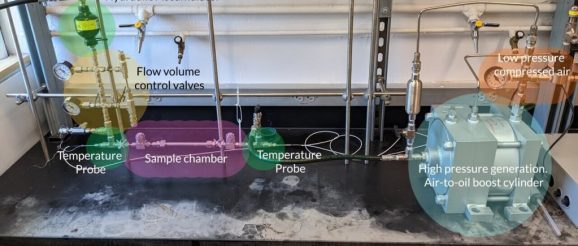
The team has now built a first-of-its-kind prototype that demonstrates the use of these new materials in a practical cooling system. The device has three main parts. One is a metal tube packed with the solid refrigerant and an inert liquid — water or an oil. Another piece of the device is a hydraulic piston that applies pressure to the liquid. Finally, the liquid helps transfer that pressure to the refrigerant and helps carry heat through the system.
After solving several engineering challenges, the team has shown that the barocaloric materials work as functional refrigerants, turning pressure changes into full temperature-changing cycles. “Our system still doesn’t use pressures as low as those of commercial refrigeration systems, but we’re getting closer,” says Mason. To the team’s knowledge, this is the first working cooling system using solid-state refrigerants that rely on pressure changes.
With the device now in hand, the team plans to test a variety of barocaloric materials. “We’re really hoping to use this machine as a testbed to help us find even better materials,” says Slavney, including ones that work at lower pressures and that conduct heat better. With an optimal material, the researchers believe solid-state refrigerants could become a viable replacement for current air conditioning and other cooling technologies.
