How to strengthen European industries’ leadership in vaccine research and innovation | VOX, CEPR Policy Portal
The Covid crisis has actually exposed the weak points of the United States social system compared to European social systems: in particular its failure to offer sufficient protection to individuals against the risk of falling sick or falling into hardship (Aghion et al. 2020). It likewise brought to light the mismanagement of the pandemic by the current administration, as exhibited by negligence in understanding the risk of the infection, pressing for exceedingly fast reopening of the economy, and resistance versus mask wearing and generalised testing. However, together with Congress, the very same administration has actually pursued a figured out and aggressive method to ensure US management in vaccine R&D and to secure supplies of future vaccines to US residents.
Although the European Commission just recently took the lead in negotiating advance purchase agreements with vaccine makers on behalf of the 27 member states and chose to offer loans to European biotechs taken part in vaccine development through the European Financial Investment Bank, it has actually fallen brief in matching the US effort to incentivise vaccine innovation– not just because of a lower level of monetary investment, however also a failure to ensure coordination across member states along with across the different funding plans for research study and development in healthcare (reflecting the more decentralised nature of R&D and health policies in Europe).
This is problematic because the very best way to get one’s economy back on track is to get rid of the infection. And, beside non-pharmaceutical steps (masks, social distancing, etc.), this means treatments and, firstly, vaccines. Naturally, a method that is too aggressive postures risks as well (e.g. dispute of interest from vaccine designers and reluctance in getting immunized).1 General
considerations
Regarding Covid-19 vaccines, it works to distinguish two phases: vaccine advancement, and the protecting of vaccine products as soon as a vaccine has been discovered and authorised. Both are needed to bring vaccines to the client (then one must still persuade, or ‘force’, individuals to get immunized).
Intuitively, contributing to vaccine advancement looks like a ‘humane’ action, because the entire world ought to take advantage of the arrival of one, or several, Covid vaccines. Instead, protecting vaccine materials ahead of time for one’s people seems more ‘self-centered’, specifically if restricted supply indicates denying vaccines to other countries’ residents. However, advanced agreements for shipment will encourage private entities to energetically pursue vaccine development– the 2 are intertwined.
More generally, when thinking about innovation for a ‘worldwide item’, it is natural to question the ‘optimum’ degree of competitors and coordination to quickly identify successful vaccines. We observe an intriguing mix of coordination and competition in the search for vaccines. Political authorities in China were denying the upcoming catastrophe, Chinese researchers have been really open about their research study results, which benefited the world research study community. The first vaccines that will become offered might be rapidly developed due to the fact that Chinese researchers published the genetic sequence of the infection as soon as it was deciphered. On the other hand, universities and personal firms, large and small, have been completing aggressively to ‘be the first’ in the race for a vaccine, consisting of in regards to raising funds from personal and state sources.
From the viewpoint of world well-being, the cooperation/open science part is naturally great. When it comes to the competition on vaccine advancement, things are more subtle: on the one hand, more monetary effort overall is an excellent idea to accelerate development for such a pricey disease (simply think about the expense of a lockdown). On the other hand, should we fret about money ‘wasted’ in moneying more than 100 vaccine tasks, consisting of advance structure of production centers? As gone over by Bolton-Farrell (1990 ), in “times of war”, speed is essential, and more coordination is more suitable to “fine-tuning for the most efficient option” if such an optimal option comes later on. We can, however, securely conclude that speed will not be hampered, offered the rush we observe (if anything, the threat is more about ‘cutting corners’ in exceedingly quick approval of vaccines which may not be safe and reliable enough).
The United States versus the EU
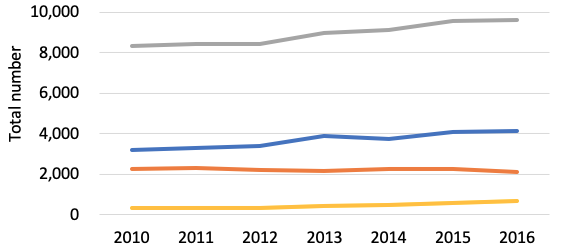
The US is a clear leader in biotech development (Figure 1 and Table 1). Additionally, it does have actually an articulated US-centric Covid method– Operation Warp Speed (OWS)– which constructs on an understanding of the complementarity between vaccine advancement and securing sophisticated materials, therefore bringing together the negotiations with private entities on the 2 phases, while relying on the combined expertise and monetary weight of existing federal instruments, in particular the National Institutes of Health (NIH) and the Biomedical Advanced Research and Advancement Authority (BARDA). This provides the US a first-mover advantage.
Figure 1 Biotechnology patents
Source: Authors’ computations utilizing OECD data. Reference nation: Innovator’s nation of home. Recommendation date: priority date.
Table 1 Biotechnology patents (per 1 million residents)
Source: Authors’ computations using OECD information.
Notes: Referral nation: Innovator’s nation of residence. Referral date: priority date.
Congress has assigned almost $10 billion to OWS, of which more than $6.5 billion was allocated to BARDA and $3 billion for NIH research study. In practice, throughout this pandemic, BARDA is offering funding to establish, to name a few, vaccines and treatments to fight Covid. Up until now, BARDA has distributed more than $11 billion among more than 40 companies to fund the development of vaccines, diagnostic, therapies, rapidly deployable capabilities, and others (Table 2).2 Table
2 BARDA’s Covid-19 medical countermeasure portfolio
Source: Authors’ calculations based upon https://medicalcountermeasures.gov/app/barda/coronavirus/COVID19.aspx.
The EU, rather, has actually pursued a less coherent technique overall, and with fewer monetary resources straight bought candidate vaccines (European Commission 2020). It is more ‘humane’ than the US in terms of vaccine advancement, pushing for worldwide cooperation, but with limited financing dedication (Tables 3 and 4). In fact, the EU makes continuous global cooperation efforts, as the Coronavirus Global Reaction exhibits. This worldwide action raised practically EUR16 billion from nations worldwide; the United States did not contribute. The EU likewise contributes through the Coalition for Upsurge Readiness Innovations (CEPI),3 an innovative partnership in between public, personal, civil and humanitarian organisations. Furthermore, the ACT-Accelerator has one vaccine pillar, COVAX, of which CEPI is co-leader together with Gavi and WHO. However, in spite of these global cooperation efforts, the EU is ‘EU-centric’ when trying to secure vaccine materials for its member states and residents. This does not sufficiently make use of the complementarity included in the process, which adds to the troublesome intricacy of financing sources (within the European budget, EIB, member states, etc).
Table 3 Coronavirus international response: Horizon 2020 pledge
Table 4 Financing from the European Commission and the European Investment Bank
Source: Authors’ estimations using data from the COVID-19 Health Financing Tracker, from The Financial expert.
Presently, there are more than 130 candidate vaccines in preclinical examination and 30 candidate vaccines in medical examination. Among these 30 prospects, 13 receive support from BARDA, CEPI and/or the EU/EIB (Table 5). 3 get support from both BARDA and CEPI (University of Oxford, Moderna and Novavax), one receives support from both CEPI and the EIB (CureVac), and one from BARDA and EIB (BioNTech). BARDA offers consistently greater funding amounts.
Table 5Collaborations to establish vaccines against Covid-19: BARDA, CEPI and EU (through EIB)
Source: Authors’ estimations based on BARDA, CEPI and Global Reaction Europe.
A restored EU support strategy to the advancement and commercialisation of ingenious innovations might be reached other areas, for example, defence-related technologies, on the design of the Defense Advanced Research Study Projects Firm (DARPA) in the United States. Interestingly, the latter has been instrumental in a number of non-defence developments. Keep in mind that we are not talking here about a restored commercial policy amounting to ‘selecting one winner’ however funding a number of completing vaccines. The DARPA model is one that mixes top-down and bottom-up: government funds are committed to funding contending groups that work on making new ‘hard technologies’ become operational. When chosen by the federal government, team leaders have complete autonomy in picking how to arrange the research study process and whom to associate with that procedure. The numerous teams will normally contend not only within Europe, but also on a more international scale, with the US, but also China and possibly Russia. So, this has to do with competition-friendly commercial policy, as advocated in Aghion et al. (2015 ).
How would a European BARDA work? While there are a number of institutional requirements to deal with, let us simply make two remarks. This is an area where signing up with forces with Britain makes sense, given its (scholastic and industrial) knowledge (the very same is real for defence). Second, one desires obviously to recognize the optimal trade-off between scale and adaptiveness/flexibility, considering that speed is typically key. This would advocate an open ‘union of the willing’, which can potentially build on insights from EU success stories (like the European Research Council, which includes non-EU partners) but must plainly prevent rigidness (e.g. juste retour, seven-year budgets) implemented by (near) unanimity ballot rules.
Competitors between Europe and the US would speed up vaccine development and supply, which can be great for the world as a whole. Naturally, ‘pressure’ on pharmaceutical business to prevent extreme profits, as well as sufficient worldwide (public and private) aid to guarantee international access, will be very crucial.
To sum up, in order to strengthen European industries management in vaccine research and innovation, we suggest the creation of a European BARDA to which EU member-states plus the UK would be welcome to participate on a voluntary basis. The BARDA design need to be adapted to make sure a choice process that is science-based and transparent, we recommend that the launch of a BARDA-type initiative must be considered in the upcoming Horizon Europe structure program for Research and Innovation. The method we recommend would be complementary to Europe’s other properties when facing epidemiological shocks, particularly an evidence-based sanitary policy and a social model that can reduce such shocks.
Aghion, P, J Cai, M Dewatripont, L Du, A Harrison and P Legros (2015 ), “Industrial Policy and Competition”, American Economic Journal: Macroeconomics 7: 1-32.
Aghion, P, H Maghin and A Sapir (2020 ), “Covid and the nature of industrialism”, VoxEU.org, 25 June.
Bolton, P and J Farrell (1990 ), “Decentralization, Duplication and Delay,” Journal of Political Economy 98: 803-826.
New York City Times (2020a), “Corporate Experts Pocket $1 Billion in Rush for Coronavirus Vaccine”, 25 July.
New York Times (2020b), “Researchers Concern About Political Impact Over Coronavirus Vaccine Job”, 2 August.
European Commission (2020 ), “EU Method for COVID-19 vaccines”, 17 June.
U.S. Department of Health and Human Being Provider (2020 ), “Discussing Operation Lightning Speed”.
1 Vaccine developers have a conflict of interest given that more positive statements help them draw in more moneying for vaccine advancement and advance production centers or, even worse, chances to cash in on efficiently timed stock sales (New York Times 2020a). Another danger is that this technique may result in unwillingness in getting vaccinated against Covid, contributing to the vaccine hesitancy issue which is already high in the United States (New York City Times 2020b).
2 Operation Warp Speed likewise collaborates other initiatives such as the NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) public-private collaboration (of which the European Medicines Company is also a partner) and the NIH’s Quick Acceleration of Diagnostics (RADx) initiative.
3 From a total of $1,280,588,290 for vaccine advancement for CEPI, the European Commission contributed with $109.2 million. The leading factors are the UK ($270 million), Norway ($213.5 million), Germany ($160.4 million), Saudi Arabia ($150 million) and Japan ($134 million), followed by the European Commission (source: The Covid-19 Health Funding Tracker from The Economist).
