Innovation Approval Edwards Lifesciences- China Med Device
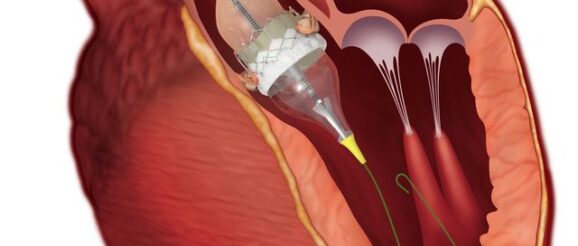
NMPA granted Innovation Approval to Edwards Lifesciences’ Transcatheter Aortic Valve System on June 8. It is the 14th device obtained such approval in 2020.
Balloon Technology
The product includes transcatheter aortic valve, transcatheter aortic valve delivery system, catheter sheath kit, transfemoral balloon catheter, and valve presetting device. The valve consists of bovine pericardial leaflets, cobalt-chromium alloy frame, and PET inner and outer skirts. It is intended for high-risk or contraindicated surgical procedures on patients with severe calcified autologous aortic valve stenosis.
NMPA sates in the approval notice that “the product adopts balloon expansion technology. The valve frame can provide high radial support force, while the low valve frame height is adapted to the surrounding anatomy, and the risk of pacemaker implantation is reduced; the paravalvular regurgitation is decreased through the outer skirt design. The incidence of paravalvular leaks is expected to be lower in clinical applications.”
Overseas Clinical Data
For clinical evaluation, Edwards Lifesciences went through the pathway of Overseas Clinical Data Acceptance.
The applicant provided overseas PIIS3HR cohort clinical trial data. The overseas data is supported by clinical trial data and literature review of the device and its similar predicates, which demonstrated that the genetic differences do not pose safety and efficacy issues.
We have the NMPA Evaluation Reports for 72 Innovation Devices granted since November 2017. For our comments on 2019 reports, please click .
Key Takeaways for Overseas Manufacturers
Only medical devices granted Innovative Device Status (not approved yet) by NMPA in 2019 were imported. Since domestic and overseas manufacturers have equal opportunity for this program, U.S. and European companies may not be taking full advantage.
Of the 39 innovation device status in 2019, only eight (just about 20%) were imported devices. One of those is Cardiovascular Systems, a company represented by China Med Device, LLC. China Med Device also conducted clinical trials for United Imaging’s CT-Linac (Click ), a combination of CT and accelerator for cancer radiotherapy. NMPA granted Innovation Approval to CT-Linac in December 2018. Email [email protected] to see if you are qualified to apply.
Overseas manufacturers have to bear in mind that:
China Med Device has translated “Innovation Approval Procedure for Medical Devices” and other official NMPA documents into English as a service for its clients. Email [email protected] for details.
