Magnetically steerable bacterial biohybrid microrobots – Innovation Toronto
Scientists add artificial components to bacteria for better control and an extra therapeutic effect in seeking and destroying tumor cells
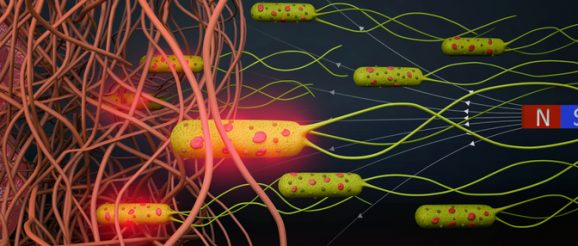
A team of scientists in the Physical Intelligence Department at the Max Planck Institute for Intelligent Systems have combined robotics with biology by equipping E. coli bacteria with artificial components to construct biohybrid microrobots. Firstly, as can be seen in Figure 1, the team attached several nanoliposomes to each bacterium. On their outer circle, these spherical-shaped carriers enclose a material (ICG, green particles) that melts when illuminated by near infrared light. Further towards the middle, inside the aqueous core, the liposomes encapsulate water soluble chemotherapeutic drug molecules (DOX).
The second component the researchers attached to the bacterium is magnetic nanoparticles. When exposed to a magnetic field, the iron oxide particles serve as an on-top booster to this already highly motile microorganism. In this way, it is easier to control the swimming of bacteria – an improved design toward an in vivo application. Meanwhile, the rope binding the liposomes and magnetic particles to the bacterium is a very stable and hard to break streptavidin and biotin complex, which was developed a few years prior (https://www.nature.com/articles/s41598-018-28102-9) and comes in useful when constructing biohybrid microrobots.
Figure 1: Bacterial biohybrids carrying nanoliposomes (200 nm) and magnetic nanoparticles (100 nm). Nanoliposomes are loaded with chemotherapeutic DOX and photothermal agent ICG, and both cargoes are conjugated to E. coli bacteria (2 to 3 µm in length) via biotin-streptavidin interactions. Akolpoglu et al., Sci. Adv. 8, eabo6163 (2022).
E. coli bacteria are fast and versatile swimmers that can navigate through material ranging from liquids to highly viscous tissues. But that is not all, they also have highly advanced sensing capabilities. Bacteria are drawn to chemical gradients such as low oxygen levels or high acidity – both prevalent near tumor tissue. Treating cancer by injecting bacteria in proximity is known as bacteria mediated tumor therapy. The microorganisms flow to where the tumor is located, grow there and in this way activate the immune system of patients. Bacteria mediated tumor therapy has been a therapeutic approach for more than a century.
For the past few decades, scientists have looked for ways to increase the superpowers of this microorganism even further. They equipped bacteria with extra components to help fight the battle. However, adding artificial components is no easy task. Complex chemical reactions are at play, and the density rate of particles loaded onto the bacteria matters to avoid dilution. The team in Stuttgart has now raised the bar quite high. They managed to equip 86 out of 100 bacteria with both liposomes and magnetic particles.
The scientists showed how they succeeded in externally steering such a high-density solution through different courses. First, through an L-shaped narrow channel with two compartments on each end, with one tumor spheroid in each. Second, an even narrower set-up resembling tiny blood vessels. They added an extra permanent magnet on one side and showed how they precisely control the drug-loaded microrobots towards tumor spheroids. And third – going one step further – the team steered the microrobots through a viscous collagen gel (resembling tumor tissue) with three levels of stiffness and porosity, ranging from soft to medium to stiff. The stiffer the collagen, the tighter the web of protein strings, the more difficult it becomes for the bacteria to find a way through the matrix (Figure 2). The team showed that once they add a magnetic field, the bacteria manage to navigate all the way to the other end of the gel as the bacteria had a higher force. Because of constant alignment, the bacteria found a way through the fibers.
Once the microrobots are accumulated at the desired point (the tumor spheroid), a near infrared laser generates rays with temperatures of up to 55 degrees Celsius, triggering a melting process of the liposome and a release of the enclosed drugs. A low pH level or acidic environment also causes the nanoliposomes to break open – hence the drugs are released near a tumor automatically.
“Imagine we would inject such bacteria based microrobots into a cancer patient’s body. With a magnet, we could precisely steer the particles towards the tumor. Once enough microrobots surround the tumor, we point a laser at the tissue and by that trigger the drug release. Now, not only is the immune system triggered to wake up, but the additional drugs also help destroy the tumor,” says Birgül Akolpoglu, a Ph.D. student in the Physical Intelligence Department at MPI-IS. She is the first author of the publication titled “Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery” co-led by former postdoctoral researcher in the Physical Intelligence Department, Dr. Yunus Alapan. It was published in Science Advances on July 15, 2022.
“This on-the-spot delivery would be minimally invasive for the patient, painless, bear minimal toxicity and the drugs would develop their effect where needed and not inside the entire body,” Alapan adds.
“Bacteria-based biohybrid microrobots with medical functionalities could one day battle cancer more effectively. It is a new therapeutic approach not too far away from how we treat cancer today,” says Prof. Dr. Metin Sitti, who leads the Physical Intelligence Department and is the last author of the publication. “The therapeutic effects of medical microrobots in seeking and destroying tumor cells could be substantial. Our work is a great example of basic research that aims to benefit our society.”
