Powering Life Sciences Innovation


Secure application delivery at speed your business demands
POWERING LIFE SCIENCES INNOVATION

ACCELERATE DIGITAL HEALTH
As more people take personal charge of their health and wellness today, care is no longer limited to the hospital, doctor’s office, or lab. Digital has become a key part of life sciences and medical device offerings as the delivery of data and diagnostics takes a “healthcare everywhere” approach.
An increased number of internet-connected devices can generate, collect, analyze and transmit critical health data. Without secure, high performance device connectivity and the capacity to draw actionable insights from this data, the potential advantages are often lost.

GET DIGITAL RIGHT
Infostretch is a digitally native company that helps Life Sciences, Healthcare and Medtech companies rethink their business processes, modernize patient engagement, and leverage digital technology to create sustainable competitive advantage.
We leverage AWS cloud to help companies get their digital innovations to market faster, with higher service levels and less risk. We adhere to a disciplined continuous review process to deliver secure and compliant solutions following industry standards and regulations like HIPAA, GDPR, FDA and FHIR.
Headquartered
in Silicon Valley
Additional Locations:
New York, Chicago,
London and India
Backed by Goldman Sachs
& Everstone Capital
Security & Compliance As IoMT brings expansive connectivity and data availability, continuous patient monitoring is the new norm. This puts both invasive and non-invasive connected devices at risk of data breaches as well as compliance penalties. Infostretch helps companies with Validation and Verification for regulatory and compliance adherence to minimize such risks.
Telemedicine & Virtual Care On-demand and remote health have exploded due to the global pandemic and are being widely supported by federal and state legislators, as well as payors and providers. As part of this trend, we at Infostretch see IoMTs enabling more and more asynchronous telemedicine practices– where health data can be transmitted continuously without the need for patients to meet with care providers in person.
Interoperability With the increased focus on remote patient care, and the desire to reduce overall diagnostic and treatment costs, there is a critical need for high levels of system interoperability to provide real-time data access. Standards like FHIR promise to provide modern and web-based suites of API technology. Infostretch offers solutions across product lifecycle while adhering to industry standards.
Patient-Centric Care & Engagement Modern patients are more informed than ever about their health, creating a paradigm shift from clinical-centric to patient-centric care. Advances in technology and utilization of patient data plays an important role in understanding more about patients and their needs to design and offer patient-centric solutions. Infostretch offers expertise in technology, health apps, SaMD, and IoMT.
Guided Digital Journey MedTech and Lifesciences companies expect partners to add value with their industry, tech and compliance expertise at every step of the digital journey. Infostretch delivers for its clients with its deep domain knowledge, expertise with the latest digital technologies, and highly tailored solution constructs.
Digital Therapeutics (DTx) As patients take more responsibility for their health, they rely more on Digital Therapeutics (DTx) and wearables. These devices must be easy to use, provide personalized experiences, and address regulatory compliance requirements. This creates the need for new device development and efficient upgrade paths for existing devices. Infostretch offers Clinical Trial Platforms which enable faster time to market while assuring compliance.
- Medical Device Development
- Health Apps
- Compliance Management
- HDAP (Healthcare Data Analytics Platform)
- Digital Therapeutics
- Patient Engagement
- Medical Devices
- HealthTech
- Biopharma
- Genomics
- Healthcare Providers (HCP)
- Payers
- Life Sciences Start-ups
- Clinical Research Organizations (CRO)
OFFERINGS ALIGNED TO PRODUCT LIFECYCLE
Accelerate the pace and success of new products – from concept to market with Infostretch.
Get ready for concept validation and initial observational studies
- Product strategy and roadmap consultancy engagement
- MVP development
- Product requirement validation
- Product effectiveness validation
- Lab-as-a-Service
- Platform for observational study automation
Design and build digital ecosystem to enable clinical trials
- BLE connectivity kit
- Digital ecosystem from firmware to cloud and visualization
- Algorithm development, edge analytics
- FDA development processes
- Software as a Medical Device (SaMD) development
- Data Interoperability (FHIR) Implementation
Design verification and test automation
- Device simulation based automation
- Design verification plan
- Document automation with FDA design verification processes
- Clinical trial management platform
- FDA auditing documentation and consultancy
Software development for end-to-end integrated devices
- Portals for providers, patients and clinicians
- Data analytics and visualizations
- Remote device management omplementation
- Firmware release and continuous testing pipeline
- Integration with partner ecosystems
Post market adjustments and preparing for scale
- Device releases management
- Incremental development and customization
- Post market adjustment verification and validation
- Internal auditing and documentation
- Support maintenance
How we
deliver

Pre-packaged medtech solutions and accelerators for faster time-to-market
Tailored offerings aligned with medtech product lifecycle
Deep technology and medtech domain expertise
Agile global teams with HIPAA and AWS experts
Proprietary methodology and frameworks
Progressive delivery
DELIVERING INNOVATION AND BUSINESS VALUE TO CLIENTS WITH MEDTECH AND IOMT FRAMEWORK
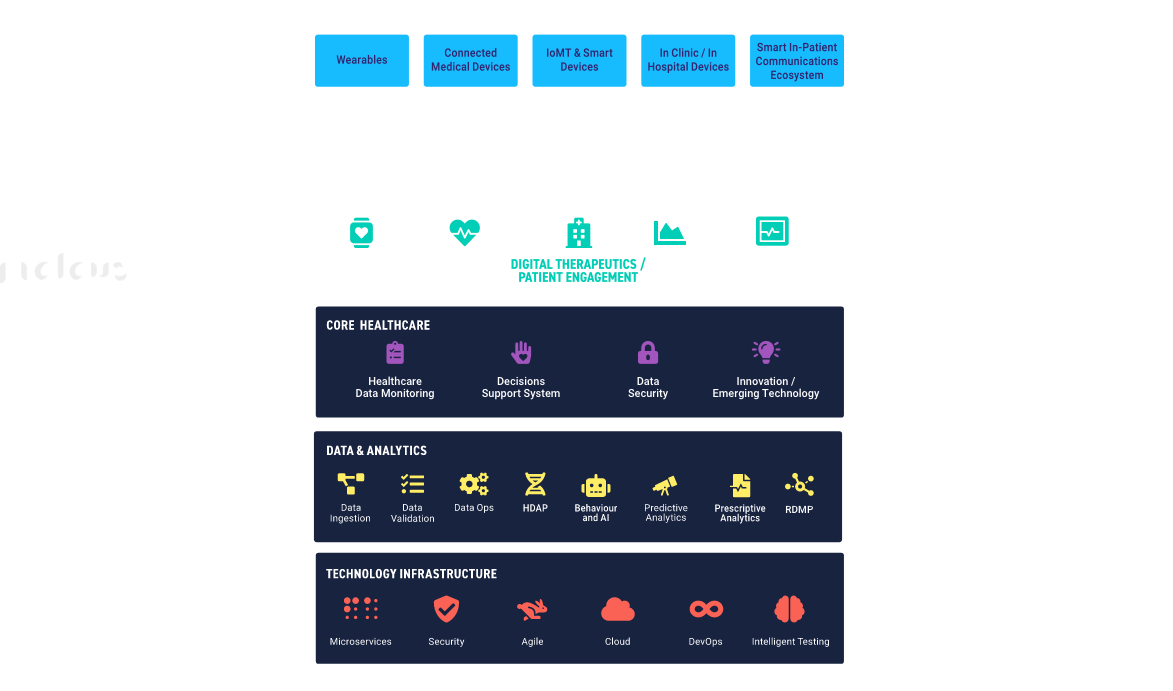
- Healthcare Data Analytics Platform (HDAP) Harness healthcare Data for a competitive advantage
- Remote Device Management Platform (RDMP) Simplify the monitoring and managing of critical IoT devices remotely
- Data Dip – Data Pipeline Validation
- Infostretch Startup Cloud Activate
- Infostretch AWS WAR (Well-Architected Review) Assessment


Certified Architects at Infostretch


The post Powering Life Sciences Innovation first appeared on Digital Engineering, Digital Technology Solutions & Services Firm – Infostretch.


