Q&A: A handheld diagnostic platform for COVID-19 molecular testing – Med-Tech Innovation | Latest news for the medical device industry
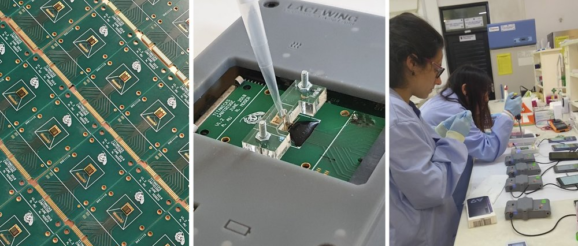
Ian Bolland caught up with Dr. Pantelis Georgiou, reader in Biomedical Electronics at Imperial College London, to discuss the collaboration between Imperial and 3D Systems as they developed a handheld diagnostic platform called Lacewing for molecular testing of COVID-19.
Lacewing is able to identify the virus that causes COVID-19 (SARS-CoV-2) in under 30 minutes. Results are also synchronised to the cloud for epidemiological surveillance and mapping of the outbreak.
More than 100 units will be produced as a first batch for validation in June at Hammersmith Hospital in London. Production will then be increased in the next six months in order to provide over 1,000 tests per month to test front-line healthcare staff and hospitalised patients as a second stage of validation.
Ian Bolland: Tell us about the work that went into making the test.
Pantelis Georgiou: For the past decade, my group at Imperial College London has been focussing on developing lab-on-chip systems based on semiconductor technology, bringing the revolution of computers and smartphones to chemical sensors. Having focussed on diagnostic technology in the past few years and built a multidisciplinary team between electrochemistry, molecular biology and microfluidics, we have developed Lacewing, a portable platform for molecular diagnostics of infectious diseases. The system is based on a cartridge housing a microchip with over 4,000 sensors, detecting in real-time the amplification of pathogenic nucleic acids (DNA or RNA) from a patient. The cartridge is plugged into a handheld device connected to a smartphone to display results in under 30 minutes and synchronise to the cloud for real-time tracking of outbreaks. Last year, the team demonstrated this technology for the detection of dengue in Taiwan and malaria in Ghana. In line with the UK plan for running 100,000 tests per day, we are now focusing all our efforts on achieving accurate COVID-19 diagnostics to face the current pandemic.
IB: How was it built in terms of its manufacture, including materials?
PG: While the internal electronics of Lacewing are all manufactured using traditional methods, similar to those used to make your phone, the rest of it, including the case and microfluidic components, are currently being 3D printed using the advanced manufacturing “Figure 4” platform from 3D Systems. Its speed, resolution, and diverse range of bio-compatible resins make it the perfect tool for our in-house manufacturing of key components.
IB: How does it compare to other tests for COVID-19, especially those that are widely known to the public?
PG: Given that Lacewing is a nucleic acid-based test, it’s more accurate and sensitive than the currently more wide-spread antibody-based tests. Additionally, unlike antibody tests, our test only shows a positive result if there is an active infection in the patient. Finally, our device not only has built-in cloud connectivity and smartphone integration for disease tracking, but it also does all this at a fraction of the cost of competing lab-based bench-top qPCR systems. Not to mention, it fits comfortably in the palm of your hand.
IB: The 30-minute turnaround. Is that quicker than most tests? How do you go about achieving a result in that time frame?
PG: One of our novelties lie in the use of rapid and specific isothermal amplification chemistries, in particular LAMP (loop mediated isothermal amplification). Combined with thousands of accurate sensors on the chip, this shows detection of SAR-CoV-2 RNA in a faster way than conventional methods.
IB: After it has been used in Hammersmith Hospital, which other locations is the test likely to be despatched to?
PG: Our first large-scale validation will be run at Charing Cross hospital in the next six months to diagnose front-line staff and patients. This will happen while we scale up our technology to 1000 tests per month. Beyond that, we are looking to more permanently establish our test within the NHS for diagnosis of COVID-19. We are also looking past the current pandemic, addressing other global health problems which have been our focus in the past years, including dengue fever, malaria and tuberculosis.
IB: Is there scope for this test to be used at home?
PG: Our current vision is to take molecular diagnostic out of the laboratory and into the community, however, we will need to validate its use for routine patient care before deploying it at homes.
IB: Anything else to add?
PG: Whilst the majority of our effort is in addressing the needs of the current pandemic locally with COVID-19 we mustn’t forget there is a huge unmet need for dealing with the control of infectious diseases in developing countries. Our technology is fast, portable and scalable, therefore we are ready to address this. We have planned validation studies looking at malaria and tuberculosis in Africa, and dengue in Asia. We are also addressing the need for better diagnostics to combat antimicrobial resistance and have recently showcased how we can rapidly respond to outbreaks in drug resistant infections through the detection of new emerging colistin resistance (mcr-9).
