Viewpoint: EU gene-editing regulations requiring traceability and labeling to ‘protect co-existence’ with organic crops could stop innovation in its tracks – Genetic Literacy Project
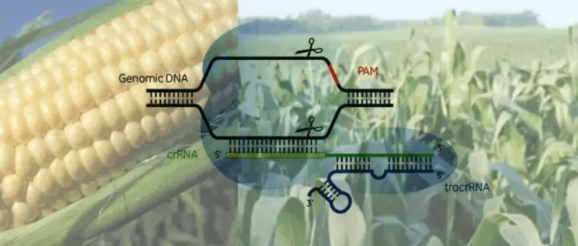
Major new developments in gene editing are now taking place with increasing frequency, as the world looks to harness the potential of genetic innovation to tackle urgent global challenges of food security, improved nutrition, climate change and pressure on finite natural resources of land, energy and water.
Just in the past couple of months, for example, the Canadian Government confirmed that gene edited crops without foreign genes will be regulated in the same way as conventionally bred varieties, and the UK Parliament approved new legislation in England which removes gene edited, or ‘precision bred’, plants and animals from the scope of restrictive GMO rules. In doing so, they joined a growing list of countries around the world seeking to encourage the use of these more precise breeding methods, including the United States, Japan, Australia, Argentina and Brazil.
Over the same period, the Chinese Government approved its first gene edited food crop, a soybean high in healthy oleic acid, the Philippines approved a gene edited ‘non-browning’ banana designed to reduce food waste, and the US authorities cleared a new type of mustard greens, gene edited for reduced bitterness and improved flavour.
Here in Europe, we continue to see major research breakthroughs in these technologies, including the recent announcement that researchers at Wageningen University in the Netherlands have used CRISPR/Cas gene editing technology to make potato plants resistant to late blight disease caused by Phytophthora infestans without inserting foreign DNA in the potato genome. It is hard to overstate the potential significance of this breakthrough, not only in safeguarding harvests from a devastating fungal infection, but also in reducing the need for pesticide sprays.
As the pace of these exciting developments accelerates around the world, a key question set to be answered over the coming months is whether Europe will join in, or remain locked out?
The European Commission is preparing to publish its long-awaited proposal for future regulation of the products of new genomic techniques (NGT), which are currently classified as GMOs in line with a European Court ruling dating back to July 2018.
In a study following this ruling the Commission concluded that the EU’s 20-year-old GMO rules are ‘not fit for purpose’ to regulate these new breeding methods, largely because those regulations were put in place years before gene editing technologies were even dreamt of.
But will the Commission’s proposal follow other countries in determining that NGT plant products which could have occurred naturally or been produced by conventional means should be regulated in the same way as their conventionally bred counterparts? Or will it succumb to the anti-science lobby, imposing GMO-style traceability, labelling and coexistence obligations for these conventional-like NGTs, which will not only deter innovation and cement the EU’s future as a museum of agriculture, but also risk trade-related challenges as gene editing becomes one of the default delivery models for global crop genetic improvement?
Earlier this month, 20 European value chain organisations, including Euroseeds, signed a joint open letter urging the Commission to treat conventional-like NGT plants in the same manner as their conventionally bred counterparts to avoid regulatory discrimination of similar products.
In the letter, all 20 organisations – representing EU farming, food and feed processing, plant breeding, scientific research and input supply organisations – underlined their commitment to transparency and information sharing to support customer and consumer choice.
Following the recent example of Canada, which has introduced a registry for gene edited plant varieties to ensure transparency and choice, the joint letter points out that national variety lists and the European Common Catalogue could be used to provide freedom of choice to farmers and growers, and allow value chains wishing to avoid the use of conventional-like NGT plants in their production to do so. Already today, for example, some private organic certification schemes exclude plant varieties bred using certain exempted methods of genetic modification such as cytoplast fusion. These private standards are observed, and the respective value chains co-exist, without the need for a specific regulatory framework, but through varietal information provided by the seed sector.
However, transparency does not necessarily imply a requirement for traceability (and/or labelling). Transparency stands at the beginning of value chains and, as such, does not disrupt food chain operations and product flows but provides freedom of choice for farmers and growers. A requirement for mandatory labelling of one particular breeding method would not only incur additional costs within the supply chain, but could also erroneously be perceived by some consumers as a warning statement and so discriminate unfairly against conventional-like NGT products. This in turn could prevent the potential of NGT plants to contribute to sustainable agricultural production and food security from being realised.
Where NGT plant products could equally have been produced using other conventional breeding methods (which are not subject to a mandatory labelling requirement), it would also constitute a breach of the fundamental principles of non-discrimination of like-products and factual information under General Food Law.
The joint value chain letter also highlighted the challenges of detection and identification of NGT plant products for market control and enforcement purposes. Since it is not technically possible to distinguish how the genetic change in a conventional-like NGT plant occurred (because it is conventional-like!), it is highly unlikely that laboratory tests would ever be able to detect and identify the presence of NGT-derived plant products in food or feed entering the EU market, creating enforcement issues and legal uncertainty for operators. The EU regulatory system risks losing trust if it is unenforceable and, with this, becomes vulnerable to fraud.
Any mandatory traceability or segregation requirements (eg paper trail systems) for technically similar products would bring significant costs and logistical burdens for operators, which are not aligned with current food trade and processing operations, and as such would represent a further, unjustified barrier to the adoption of NGT plants in the EU.
Finally, in relation to the coexistence of farming systems and international trade, the joint letter points out that, today, EU regulations do not impose coexistence measures between conventional and organic farming, even though some organic farming standards already exclude plant varieties from certain non-regulated-GMO breeding methods. Similarly, the US, with which the EU has agreed equivalency schemes for organic food, does not impose specific coexistence measures between organic and conventional farmers (including for conventional-like NGT products). This has the obvious advantage for US organic growers and food producers that such food will also be accepted as organic in the EU. In sharp contrast, always imposing risk assessment and traceability plus labelling requirements (as well as coexistence measures) for conventional-like NGT plants and products would be incompatible with organic standards in third countries like the US. This would endanger well-established equivalency standards and international organic value chains.
In short, imposing traceability and labelling requirements, and coexistence measures that place specific obligations on farmers growing conventional-like NGT varieties, would have negative implications for the competitiveness of the EU agri-food value chain as well as the enforceability of regulations.
It would also be at odds with the EU’s guiding regulatory principles of practicality, proportionality and non-discrimination.
Our policy-makers have a unique opportunity to embrace and enable the use of these more precise breeding technologies in European agriculture, and to improve prospects for delivering the sustainability objectives set out in the EU’s Green Deal.
Is the EU ready to join the global gene editing revolution, or will we remain locked in a political and regulatory time warp?
Petra Jorasch holds a PhD in plant molecular biology from the University of Hamburg. She is an internationally recognised science, communication and industry advocacy expert with more than 20 years of experience in and a deep knowledge of the relevant policy frameworks for seeds, plant science and breeding, access and use of plant genetic resources as well as relevant intellectual property protection systems. Follow Petra on Linkedin
